It's WAY too early to call this Insulin Pump an Artificial Pancreas
The diabetic internet and lots of mainstream news agencies are abuzz about the new insulin pump from Medtronic. Poorly written news articles that are effectively regurgitations of the Medtronic Press Release have exciting headlines like this:
- FDA approves artificial pancreas you can wear
- Medtronic's Artificial Pancreas Approved for US Diabetics
- FDA Approves First Artificial Pancreas For Patients With Diabetes
Other news outlets have slightly better headlines like
But then ruin it with vague subtitles that are missing important context:
- FDA approved the company’s automated insulin delivery system.
This is Step 1, possibly Step 0.
TO BE CLEAR. This new Medtronic 530G pump is NOT an artificial pancreas. It is an insulin pump, similar to the very model I'm wearing right now. It is paired with a revision of Medtronic's CGM (Continuous Glucose Meter) system and it does one new thing.
This new pump will turn off if you ignore its alarm that you may be having a low blood sugar.
Read it again, I'll wait.
Note the JDRF chart above describing the steps we need to towards a true artificial pancreas. This new 530G from Medtronic is arguably Step 1 in this 6 step process. It's the first step of the first generation.
But wait, doesn't your pump just handle things for you? You don't have to stick your fingers anymore, right? Wrong.
Let's stop and level set for a moment. Here's a generalization of your day if you're not diabetic.
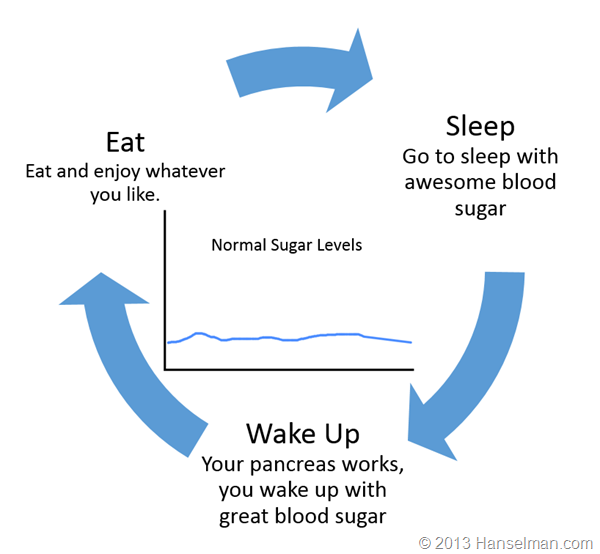
Here's what a Type 1 diabetic (like me) does:
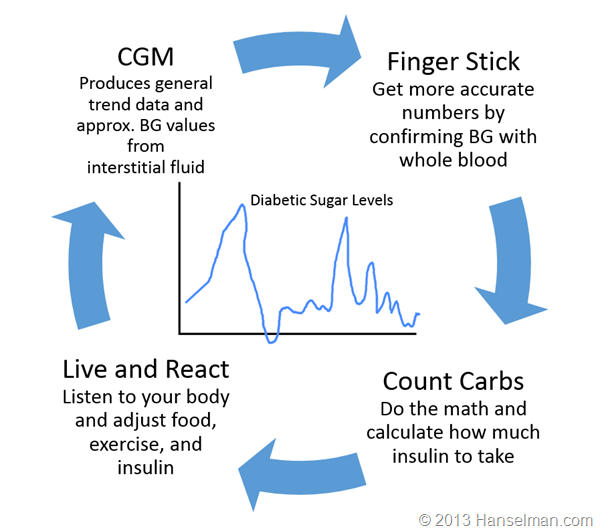
If I get this new pump that news outlets are incorrectly calling an artificial pancreas will anything in this cycle change? No.
There's NOTHING automatic here. I want to make that clear. Today's insulin pumps are NOT automatic. I set them manually, I tell them what to do manually. Yes, they "automatically deliver insulin as I sleep" but only because I told it to. If I eat and do nothing, I WILL get high blood sugar and today's insulin pumps will do exactly NOTHING about it.
If I only make decisions about insulin dosage based on my CGM then I WILL eventually get in trouble because today's CGMs are demonstrably less accurate than finger sticks. And, here's the kicker, finger sticks aren't even that accurate either.
Even more insidious is the issue of lag time. Medtronic's last generation of CGM lagged by 20 to 30 minutes BEHIND a finger stick. That meant I was getting "real time values" that in fact represented my blood sugar in the past. It's hard to make reliable altitude changes in your plane if your altimeter shows your altitude a half hour ago.
The Medtronic Press Release says that this new Enlite Sensor is 31% more accurate. I hope so. I personally continue to use a Medtronic 522 pump (this new one is the 530G) but I have given up on Medtronic's CGM in favor of a Dexcom G4. I am thrilled with it. The G4 has about a 5 minute lag time and is astonishingly accurate.
NOTE: I have no personal or investment relationship with either Dexcom or Medtronic. I am not a doctor or a scientist. I write this blog post with the expertise of someone who has been a Type 1 Diabetic for 20 years, a user of a Medtronic Pump for 15 years, a user of a Medtronic CGM for 4 years, and more recently a user of a Dexcom G4 for a year. My most recent A1C test was 5.5 putting my blood sugars at near non-diabetic levels on average. TL;DR - I'm a very good diabetic who uses the best available technology to keep me alive as long as possible.
I am extremely disappointed in the lack of research, due diligence and basic medical common sense in these articles. If you are a Type 1 Diabetic or have someone in your life who is, do the research and the reading and please spread the word so people can make informed decisions.
Related Reading
- The Sad State of Diabetes Technology in 2012
- Scott's Diabetes Explanation: The Airplane Analogy
- YOUTUBE: How my diabetes equipment works
About Scott
Scott Hanselman is a former professor, former Chief Architect in finance, now speaker, consultant, father, diabetic, and Microsoft employee. He is a failed stand-up comic, a cornrower, and a book author.
About Newsletter
From people/reports/blogs it seems Medtronic's CGM is way slower to react in comparison to Dexcom G4 and that is a key reason for the low glucose insulin off on the Veo. It also seems that Medtronic CGM wearers rarely get more than a week out of their sensors, which is a big thing for us Brits who generally have to self fund CGM sensors.
In comparison a Dexcom G4's lag time is a lot less, it's (allegedly) more accurate and more important for me it seems easy to get over 2 weeks on a sensor, I know many who regularly get 5 weeks. Hence the reason for the Animas Vibe.
I'll wait until we get to the second generation phase before I get too excited. Having said this I believe any pump is a great step forward in comparison to the multiple daily injections my daughter was on back in June.
Seems a variation of the Dunning-Kruger effecrt
http://en.wikipedia.org/wiki/Dunning%E2%80%93Kruger_effect
i'm a type 1 diabetic since 4 years (now 4 months with a pump, this thing is good!).
Because of that a colleague sends me today the following article:
https://myglu.org/articles/bionic-bill-day-1-falling-in-love
I would love to test this piece of technology..
Keep up the good work!
I do volunteer for JDRF in Nebraska, and spread the word where I can! http://jdrf.org/about-jdrf/fact-sheets/research-funding-facts/
Really appreciate your great posts.
I won't even wear a CGM until they get the calibration down to a few checks every day...
With the number of dollars spent on research in the diabetes space and the cost of these devices and aftermarket supplies ($1B insulin pump market), I find it difficult to understand the lack of advancement. Not to downplay the hard work of those involved but, and this may be ignorance and frustration on my part, I feel as though stem cell research and/or gene therapy will lead to an answer before an artificial pancreas (me being extreme).
I had no idea that diabetics had to go through that process and pain.
I'm actually surprised that in 2013 there isn't a better way to deal with this. Forgive my ignorance if that is a stupid thing to say.
My wife has been diagnosed less than a year ago as type one. It was a big change in our lives. But if one good thing came out of it, it was that I realised how much I love her and how much I took her for granted. I wish she could stop destroying her pancreas and I would happily give her mine.
Thanks for the info.
Too much hype, and so many people take the "Cliffs Notes" version that the media puts out as cold, hard facts, without checking for themselves.
Appreciate this post.
Kind Regards,
Dave
I was lucky enough to be part of the study (been type 1 since 1984). I've used a medtronic sensor in the past, but the insertion device (the blue tube-thing held at an angle) was brutal. And having to do that every three days wasn't appealing.
I was pleased with the Enlite sensor insertion device, a much easier process. Also, the 'tape' provided with the sensors kept it on for the full 6 days.
My A1C improved over the course of the study period. I attribute it to the fact that having my blood sugar number so easily accessible kept me from 'forgetting' I was a diabetic. True, there is a delay, but it's better than not having a sensor. I'll have to look into the G4, thanks for that.
The alarm woke me up a few times for lows, but I caught it before it stopped the basal delivery.
I agree the headlines are misleading. But hopefully future studies get me closer to having a pancrease in my pocket..
...why nobody uses some hardware ie dexcom G4, Omnipod, polar actocity tracker?
a seperat cgm sensor like the G4
a small pump like the omnipod
controlled by only 1 controll-device - maybe a smartphone
.and. include some activity sensors in one of the devises you have to wear.
This device could to everything except count carbs...
Thanks for publishing this. I've been getting calls from Medtronic of late about moving to the new pump/sensor system and it's nice to have a independent view.
One question: In your experience does the improved accuracy of the Dexcom outweigh the added hassle of having to lug around another chunk of tech on your belt? I understand that you've been using the Dexcom for over a year now, and wondered if you still thought well of it.
FYI I was diagnosed almost 50 years ago, and while the changes in tech are impressive, it's still a pain to do the diabetic gear dance. In the old days, they used glass syringes with needles that had to be re-sharpened(!) and urine tests for blood sugar, and there have been big changes since then, but the gear hassles are still with us.
Btw, I've been following your blog for a few years as a developer. I've spent the past couple of hours digging through your posts on diabetes and am quite literally motivated to manage my bg better. Thanks for that!
-k
Seriously if Medtronic would just release the super secret wireless pump that has supposedly been in development for the last 4 years they could actually own the business landscape if the device were capable of handling more than a 3 day supply of insulin, that's the roadblock of all these companies, OMNI-POD has a wireless unit but same deal, unit change out every 3 days or so and it's just too dang large on your body and for me it's simply "NOT GOOD ENOUGH".
(Please delete if you feel this is spam)
At the age of 45 I know diabetes (Type I myself for the last 25 years). Let me tell you about the injections I give...
7 AM = 15 to 20 units of R
12 Noon = 15 to 20 units of R
5 PM = 15 to 20 units of R
If at any time the blood sugar appears over 300 I up the dose.
At night, I give 15 to 20 units of N.
I noticed sunlight helps a lot!
I noticed exercise helps a lot!
I noticed that proper vegan foods help a lot too!
I noticed a proper family helps at times too.
I keep praying for a termination to the disease upon myself, but I sometimes feel weak in my prayers, but I keep going nonetheless. Thank you for your video. I enjoyed it greatly. I keep my faith in my/our Father. I hold no desire to use a pump, but I enjoyed the video greatly. Again, thank you.
Don't know if you've seen this, but a PhD. just published a comparison of the Medtronic and Dexcom systems, which makes for very interesting reading. He seems to agree with you that the Dexcom comes out on top accuracy-wise. But I'll admit I'm still kind of hung up on the ease of using the Medtronic system, given the fewer numbers of tech one has to hang on one's belt, and the easy communication between the medtronic pumps and sensors.
His post is here: http://www.tudiabetes.org/group/dexcomusers/forum/topics/report-analysis-medtronic-enlite-vs-dexcom-g4
and his report is here: http://api.ning.com/files/aoq71LIlZOBimTCXqcAyB2ecBcsOPFzbkpgald3cdssaiyWnRB2KxhZtduGDNw4Af-B2vrWHwI7C2IoIY4FmPqxLxxAe39dm/CGMreport.pdf
Sorry, but I can't figure out how to insert links. You'll have to copy and paste the URLs.
Comments are closed.
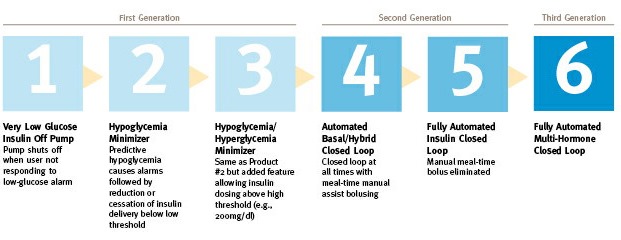

It's also worth noting that the "one new feature" that's being touted as 'artificial pancreas technology' because of some specific category that the FDA filed the approval under (https://twitter.com/thedamon/status/384420935685910529) has already been available in other countries for a couple of years.